题目
p(atm)-|||-pc c-|||-pb b-|||-pd _-|||-d-|||-pa-|||-a-|||-V(L)-|||-O V1 V2 1 mol 氦气作如图所示的可逆循环过程,其中ab和cd是绝热过程, bc和da为等体过程,已知 V1 = 16.4 L,V2 = 32.8 L,pa = 1 atm,pb = 3.18 atm,pc = 4 atm,pd = 1.26 atm,试求:(1)在各态氦气的温度.(2)在态氦气的内能.(3)在一循环过程中氦气所作的净功.(1 atm = 1.013×105 Pa) (普适气体常量R = 8.31 J· mol1· K1)
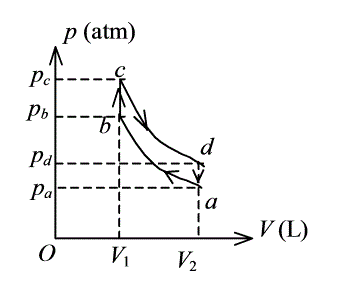 1 mol 氦气作如图所示的可逆循环过程,其中ab和cd是绝热过程, bc和da为等体过程,已知 V1 = 16.4 L,V2 = 32.8 L,pa = 1 atm,pb = 3.18 atm,pc = 4 atm,pd = 1.26 atm,试求:
1 mol 氦气作如图所示的可逆循环过程,其中ab和cd是绝热过程, bc和da为等体过程,已知 V1 = 16.4 L,V2 = 32.8 L,pa = 1 atm,pb = 3.18 atm,pc = 4 atm,pd = 1.26 atm,试求:
(1)在各态氦气的温度.
(2)在态氦气的内能.
(3)在一循环过程中氦气所作的净功.
(1 atm = 1.013×105 Pa) (普适气体常量R = 8.31 J· mol1· K1)
题目解答
答案
解:(1) Ta = paV2/R=400 K
Tb = pbV1/R=636 K
Tc = pcV1/R=800 K
Td = pdV2/R=504 K 4分
(2) Ec =(i/2)RTc=9.97×103 J 2分
(3) b-c等体吸热
Q1=CV(TcTb)=2.044×103 J 1分
d-a等体放热
Q2=CV(TdTa)=1.296×103 J 1分
W=Q1Q2=0.748×103 J 2分
题号:20644010
分值:10分
难度系数等级:4
解析
步骤 1:计算各态氦气的温度
根据理想气体状态方程 \(pV = nRT\),可以计算出各态氦气的温度。氦气的摩尔数 \(n = 1\) mol,普适气体常量 \(R = 8.31\) J·mol⁻¹·K⁻¹。
- 对于态a,\(T_a = \frac{p_aV_2}{nR} = \frac{1 \times 1.013 \times 10^5 \times 32.8 \times 10^{-3}}{1 \times 8.31} = 400\) K
- 对于态b,\(T_b = \frac{p_bV_1}{nR} = \frac{3.18 \times 1.013 \times 10^5 \times 16.4 \times 10^{-3}}{1 \times 8.31} = 636\) K
- 对于态c,\(T_c = \frac{p_cV_1}{nR} = \frac{4 \times 1.013 \times 10^5 \times 16.4 \times 10^{-3}}{1 \times 8.31} = 800\) K
- 对于态d,\(T_d = \frac{p_dV_2}{nR} = \frac{1.26 \times 1.013 \times 10^5 \times 32.8 \times 10^{-3}}{1 \times 8.31} = 504\) K
步骤 2:计算态c氦气的内能
氦气为单原子分子,其内能 \(E = \frac{3}{2}nRT\)。
- 对于态c,\(E_c = \frac{3}{2} \times 1 \times 8.31 \times 800 = 9.97 \times 10^3\) J
步骤 3:计算循环过程中氦气所作的净功
- b-c等体吸热 \(Q_1 = C_V(T_c - T_b) = \frac{3}{2}nR(T_c - T_b) = \frac{3}{2} \times 1 \times 8.31 \times (800 - 636) = 2.044 \times 10^3\) J
- d-a等体放热 \(Q_2 = C_V(T_d - T_a) = \frac{3}{2}nR(T_d - T_a) = \frac{3}{2} \times 1 \times 8.31 \times (504 - 400) = 1.296 \times 10^3\) J
- 净功 \(W = Q_1 - Q_2 = 2.044 \times 10^3 - 1.296 \times 10^3 = 0.748 \times 10^3\) J
根据理想气体状态方程 \(pV = nRT\),可以计算出各态氦气的温度。氦气的摩尔数 \(n = 1\) mol,普适气体常量 \(R = 8.31\) J·mol⁻¹·K⁻¹。
- 对于态a,\(T_a = \frac{p_aV_2}{nR} = \frac{1 \times 1.013 \times 10^5 \times 32.8 \times 10^{-3}}{1 \times 8.31} = 400\) K
- 对于态b,\(T_b = \frac{p_bV_1}{nR} = \frac{3.18 \times 1.013 \times 10^5 \times 16.4 \times 10^{-3}}{1 \times 8.31} = 636\) K
- 对于态c,\(T_c = \frac{p_cV_1}{nR} = \frac{4 \times 1.013 \times 10^5 \times 16.4 \times 10^{-3}}{1 \times 8.31} = 800\) K
- 对于态d,\(T_d = \frac{p_dV_2}{nR} = \frac{1.26 \times 1.013 \times 10^5 \times 32.8 \times 10^{-3}}{1 \times 8.31} = 504\) K
步骤 2:计算态c氦气的内能
氦气为单原子分子,其内能 \(E = \frac{3}{2}nRT\)。
- 对于态c,\(E_c = \frac{3}{2} \times 1 \times 8.31 \times 800 = 9.97 \times 10^3\) J
步骤 3:计算循环过程中氦气所作的净功
- b-c等体吸热 \(Q_1 = C_V(T_c - T_b) = \frac{3}{2}nR(T_c - T_b) = \frac{3}{2} \times 1 \times 8.31 \times (800 - 636) = 2.044 \times 10^3\) J
- d-a等体放热 \(Q_2 = C_V(T_d - T_a) = \frac{3}{2}nR(T_d - T_a) = \frac{3}{2} \times 1 \times 8.31 \times (504 - 400) = 1.296 \times 10^3\) J
- 净功 \(W = Q_1 - Q_2 = 2.044 \times 10^3 - 1.296 \times 10^3 = 0.748 \times 10^3\) J