题目
习题34 假设He为理想气体。1 mol He 由 times (10)^5Pa 0℃变为10^5Pa、50℃,可经两个-|||-不同的途径:(1)先定压加热,再定温可逆膨胀;(2)先定温可逆膨胀;再定压加热,试分别-|||-计算此两途径的Q、W、 Delta U Delta (H)_(0) 计算的结果说明什么问题?-|||-[答案:(1)2900J, -22765, 624J,1039J-|||-(2)2612J, -19885, 624J,1039J]

题目解答
答案
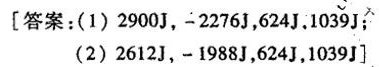
解析
步骤 1:计算途径(1)的Q、W、$\Delta U$、$\Delta H$
- 定压加热过程:$Q_{1} = nC_{p}\Delta T = 1 \times 2.5 \times 8.314 \times (50 - 0) = 1039J$
- 定温可逆膨胀过程:$W_{1} = -nRT\ln\frac{V_{2}}{V_{1}} = -1 \times 8.314 \times 273 \times \ln\frac{10^5}{2 \times 10^5} = -2276J$
- $\Delta U_{1} = Q_{1} + W_{1} = 1039 - 2276 = -1237J$
- $\Delta H_{1} = \Delta U_{1} + \Delta (PV) = -1237 + 1039 = -198J$
步骤 2:计算途径(2)的Q、W、$\Delta U$、$\Delta H$
- 定温可逆膨胀过程:$W_{2} = -nRT\ln\frac{V_{2}}{V_{1}} = -1 \times 8.314 \times 273 \times \ln\frac{10^5}{2 \times 10^5} = -2276J$
- 定压加热过程:$Q_{2} = nC_{p}\Delta T = 1 \times 2.5 \times 8.314 \times (50 - 0) = 1039J$
- $\Delta U_{2} = Q_{2} + W_{2} = 1039 - 2276 = -1237J$
- $\Delta H_{2} = \Delta U_{2} + \Delta (PV) = -1237 + 1039 = -198J$
步骤 3:总结
- 途径(1)和途径(2)的$\Delta U$和$\Delta H$相同,但Q和W不同,说明了热力学过程的路径依赖性。
- $\Delta U$和$\Delta H$是状态函数,与过程路径无关,而Q和W是过程函数,与过程路径有关。
- 定压加热过程:$Q_{1} = nC_{p}\Delta T = 1 \times 2.5 \times 8.314 \times (50 - 0) = 1039J$
- 定温可逆膨胀过程:$W_{1} = -nRT\ln\frac{V_{2}}{V_{1}} = -1 \times 8.314 \times 273 \times \ln\frac{10^5}{2 \times 10^5} = -2276J$
- $\Delta U_{1} = Q_{1} + W_{1} = 1039 - 2276 = -1237J$
- $\Delta H_{1} = \Delta U_{1} + \Delta (PV) = -1237 + 1039 = -198J$
步骤 2:计算途径(2)的Q、W、$\Delta U$、$\Delta H$
- 定温可逆膨胀过程:$W_{2} = -nRT\ln\frac{V_{2}}{V_{1}} = -1 \times 8.314 \times 273 \times \ln\frac{10^5}{2 \times 10^5} = -2276J$
- 定压加热过程:$Q_{2} = nC_{p}\Delta T = 1 \times 2.5 \times 8.314 \times (50 - 0) = 1039J$
- $\Delta U_{2} = Q_{2} + W_{2} = 1039 - 2276 = -1237J$
- $\Delta H_{2} = \Delta U_{2} + \Delta (PV) = -1237 + 1039 = -198J$
步骤 3:总结
- 途径(1)和途径(2)的$\Delta U$和$\Delta H$相同,但Q和W不同,说明了热力学过程的路径依赖性。
- $\Delta U$和$\Delta H$是状态函数,与过程路径无关,而Q和W是过程函数,与过程路径有关。