题目
容器中有氮气100g,温度为298.2K,压力为100。令该气体反抗外压为10做恒外压绝热膨胀,直至气体的压力和外压相等,试计算:(1)气体终态的温度;(2)膨胀过程气体做的功和焓变。(设氮气为理想气体,)
容器中有氮气100g,温度为298.2K,压力为100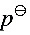 。令该气体反抗外压为10
。令该气体反抗外压为10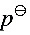 做恒外压绝热膨胀,直至气体的压力和外压相等,试计算:
做恒外压绝热膨胀,直至气体的压力和外压相等,试计算:
(1)气体终态的温度;
(2)膨胀过程气体做的功和焓变。(设氮气为理想气体,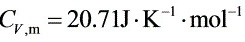 )
)
题目解答
答案
解:(1)过程图如下:

因Q=0,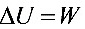 ,即
,即
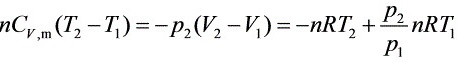
整理得 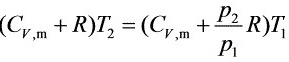
即 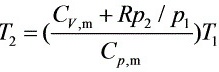
将p1=100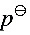 、p2=10
、p2=10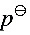 、T1=298.2K和
、T1=298.2K和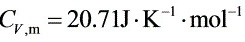 、
、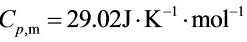
代入上式解得
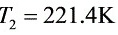
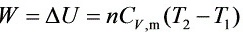
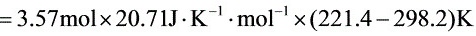
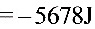
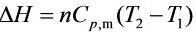
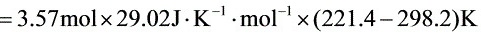
解析
步骤 1:计算氮气的摩尔数
氮气的摩尔质量为28g/mol,因此100g氮气的摩尔数为:
$n=\dfrac {100g}{28g\cdot {mol}^{-1}}=3.57mol$
步骤 2:计算终态温度
由于过程是恒外压绝热膨胀,根据绝热过程的公式,有:
${{x}_{r}m}({I}_{2}-{I}_{1})=-{p}_{2}({V}_{2}-{V}_{1})=-nR{I}_{2}+\dfrac {{P}_{2}}{{P}_{1}}nR{T}_{1}$
整理得:
$({C}_{r}m+R){T}_{2}=({C}_{r}_{r}m)+\dfrac {{p}_{2}}{{p}_{1}}R){T}_{1}$
即:
${T}_{2}=(\dfrac {{C}_{V}m+{R}_{{P}_{2}/{P}_{1}}{{C}_{P}m}{{T}_{1}}$
将p1=100①、p2=10①、T1=298.2K和${C}_{V}m=20.71J\cdot {K}^{-1}\cdot {mol}^{-1}$、${C}_{p,m}=29.02J\cdot {K}^{-1}\cdot {mol}^{-1}$代入上式解得:
${T}_{2}=221.4K$
步骤 3:计算膨胀过程气体做的功和焓变
根据绝热过程的性质,有:
$W=\Delta U=n{C}_{V}m,({T}_{2}-{T}_{1})$
$=3.57mol\times 20.71J\cdot {K}^{-1}\cdot {mol}^{-1}\times (221.4-298.2)K$
$=-56781J$
$\Delta H=n{C}_{p}m({I}_{2}-{T}_{1})$
$=3.57mol\times 29.02J\cdot {K}^{-1}\cdot {mol}^{-1}\times (221.4-298.2)K$
$=-79571J$
氮气的摩尔质量为28g/mol,因此100g氮气的摩尔数为:
$n=\dfrac {100g}{28g\cdot {mol}^{-1}}=3.57mol$
步骤 2:计算终态温度
由于过程是恒外压绝热膨胀,根据绝热过程的公式,有:
${{x}_{r}m}({I}_{2}-{I}_{1})=-{p}_{2}({V}_{2}-{V}_{1})=-nR{I}_{2}+\dfrac {{P}_{2}}{{P}_{1}}nR{T}_{1}$
整理得:
$({C}_{r}m+R){T}_{2}=({C}_{r}_{r}m)+\dfrac {{p}_{2}}{{p}_{1}}R){T}_{1}$
即:
${T}_{2}=(\dfrac {{C}_{V}m+{R}_{{P}_{2}/{P}_{1}}{{C}_{P}m}{{T}_{1}}$
将p1=100①、p2=10①、T1=298.2K和${C}_{V}m=20.71J\cdot {K}^{-1}\cdot {mol}^{-1}$、${C}_{p,m}=29.02J\cdot {K}^{-1}\cdot {mol}^{-1}$代入上式解得:
${T}_{2}=221.4K$
步骤 3:计算膨胀过程气体做的功和焓变
根据绝热过程的性质,有:
$W=\Delta U=n{C}_{V}m,({T}_{2}-{T}_{1})$
$=3.57mol\times 20.71J\cdot {K}^{-1}\cdot {mol}^{-1}\times (221.4-298.2)K$
$=-56781J$
$\Delta H=n{C}_{p}m({I}_{2}-{T}_{1})$
$=3.57mol\times 29.02J\cdot {K}^{-1}\cdot {mol}^{-1}\times (221.4-298.2)K$
$=-79571J$