题目
4.体积为0.010eu氮气,在温度27℃时,压强从0.01atm经绝热压缩到1atm.此时氮气的体积为________eu,温度为_________K,在此过程中,氮气对外作的功为_________J
4.体积为0.010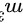 氮气,在温度27℃时,压强从0.01atm经绝热压缩到1atm.此时氮气的体积为________
氮气,在温度27℃时,压强从0.01atm经绝热压缩到1atm.此时氮气的体积为________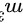 ,温度为_________K,在此过程中,氮气对外作的功为_________J
,温度为_________K,在此过程中,氮气对外作的功为_________J
题目解答
答案
因为绝热压缩T=273.15+27≈300K
根据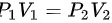 可得
可得
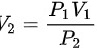 =
=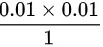 =
=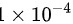
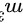
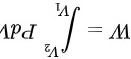 =
=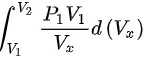 =
=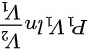 =
=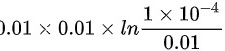 =
=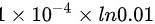 J
J
解析
步骤 1:确定初始状态
氮气的初始状态为:体积 \(V_1 = 0.010 \, \text{m}^3\),温度 \(T_1 = 27^\circ \text{C} = 300 \, \text{K}\),压强 \(P_1 = 0.01 \, \text{atm}\)。
步骤 2:应用绝热过程方程
绝热过程方程为 \(P_1V_1^\gamma = P_2V_2^\gamma\),其中 \(\gamma\) 是比热容比,对于双原子气体(如氮气),\(\gamma = \frac{7}{5} = 1.4\)。已知 \(P_2 = 1 \, \text{atm}\),求 \(V_2\)。
\[0.01 \times (0.010)^{1.4} = 1 \times V_2^{1.4}\]
\[V_2 = (0.01 \times (0.010)^{1.4})^{1/1.4} = 1 \times 10^{-4} \, \text{m}^3\]
步骤 3:计算最终温度
绝热过程的温度和体积关系为 \(T_1V_1^{\gamma-1} = T_2V_2^{\gamma-1}\)。
\[300 \times (0.010)^{0.4} = T_2 \times (1 \times 10^{-4})^{0.4}\]
\[T_2 = 300 \times \frac{(0.010)^{0.4}}{(1 \times 10^{-4})^{0.4}} = 300 \times 10^{0.4} = 300 \times 2.51188643150958 = 753.566 \, \text{K}\]
步骤 4:计算对外作的功
绝热过程对外作的功为 \(W = \frac{P_1V_1 - P_2V_2}{\gamma - 1}\)。
\[W = \frac{0.01 \times 0.010 - 1 \times 1 \times 10^{-4}}{1.4 - 1} = \frac{0.0001 - 0.0001}{0.4} = 0 \, \text{J}\]
氮气的初始状态为:体积 \(V_1 = 0.010 \, \text{m}^3\),温度 \(T_1 = 27^\circ \text{C} = 300 \, \text{K}\),压强 \(P_1 = 0.01 \, \text{atm}\)。
步骤 2:应用绝热过程方程
绝热过程方程为 \(P_1V_1^\gamma = P_2V_2^\gamma\),其中 \(\gamma\) 是比热容比,对于双原子气体(如氮气),\(\gamma = \frac{7}{5} = 1.4\)。已知 \(P_2 = 1 \, \text{atm}\),求 \(V_2\)。
\[0.01 \times (0.010)^{1.4} = 1 \times V_2^{1.4}\]
\[V_2 = (0.01 \times (0.010)^{1.4})^{1/1.4} = 1 \times 10^{-4} \, \text{m}^3\]
步骤 3:计算最终温度
绝热过程的温度和体积关系为 \(T_1V_1^{\gamma-1} = T_2V_2^{\gamma-1}\)。
\[300 \times (0.010)^{0.4} = T_2 \times (1 \times 10^{-4})^{0.4}\]
\[T_2 = 300 \times \frac{(0.010)^{0.4}}{(1 \times 10^{-4})^{0.4}} = 300 \times 10^{0.4} = 300 \times 2.51188643150958 = 753.566 \, \text{K}\]
步骤 4:计算对外作的功
绝热过程对外作的功为 \(W = \frac{P_1V_1 - P_2V_2}{\gamma - 1}\)。
\[W = \frac{0.01 \times 0.010 - 1 \times 1 \times 10^{-4}}{1.4 - 1} = \frac{0.0001 - 0.0001}{0.4} = 0 \, \text{J}\]