题目
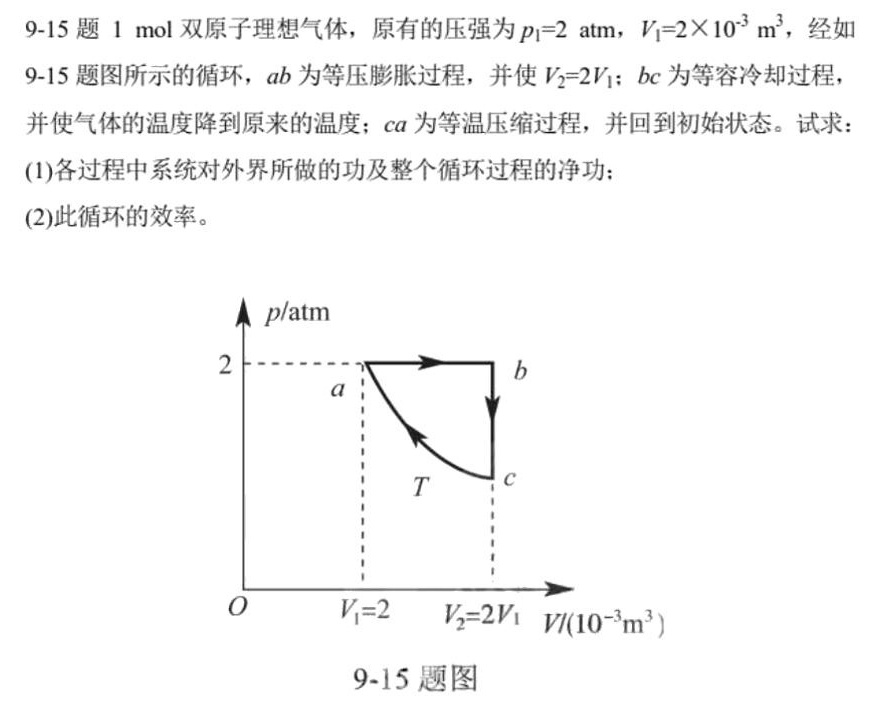
9-15 题1mol双原子理想气体,原有的压强为 _(1)=2atan , _(1)=2times (10)^-3(m)^3, 经如-|||-9-15 题图所示的循环,ab为等压膨胀过程,并使 _(2)=2(V)_(1); bc为等容冷却过程,-|||-并使气体的温度降到原来的温度;ca为等温压缩过程,并回到初始状态。试求:-|||-(1)各过程中系统对外界所做的功及整个循环过程的净功;-|||-(2)此循环的效率。-|||-p/atm-|||-2 b-|||-a-|||-T c-|||-O ({v)_(1)}=2 _(2)=2(V)_(1) _(1)((10)^-3(m)^3)-|||-9-15 题图
题目解答
答案

解析
步骤 1:计算各状态点的值
- a点:${V}_{a}=2\times {10}^{-3}{m}^{3}$, ${P}_{a}=2\times 1.013\times {10}^{5}Pa$, ${T}_{a}=\dfrac {{V}_{a}{P}_{a}}{R}=48.4K$
- b点:${V}_{b}=2{V}_{a}$, ${P}_{b}={P}_{a}$, ${T}_{b}=2{T}_{a}$
- c点:${V}_{C}={V}_{b}$, ${P}_{c}=\dfrac {1}{2}{P}_{a}$, ${T}_{c}={T}_{a}$
步骤 2:计算各过程中的功
- A→B等压过程:${A}_{1}=v{C}_{ma}({T}_{b}-{T}_{c})=\dfrac {7}{2}\times 8.31\times 48.8=1419.31J$
- B→C等容过程:${A}_{2}=0$
- C→A等温过程:${A}_{3}=vRTi\ln \dfrac {{v}_{1}}{{v}_{2}}=8.31\times 48.8\times (-0.693)=-281.0J$
步骤 3:计算整个循环过程的净功
- 整个循环过程的净功为:$A={A}_{1}+{A}_{2}+{A}_{3}=(405.2-281.0)J=124.2J$
步骤 4:计算循环效率
- 循环效率为:$\eta =\dfrac {A}{Q}=\dfrac {124.2}{1419.3}=8.75\%$
- a点:${V}_{a}=2\times {10}^{-3}{m}^{3}$, ${P}_{a}=2\times 1.013\times {10}^{5}Pa$, ${T}_{a}=\dfrac {{V}_{a}{P}_{a}}{R}=48.4K$
- b点:${V}_{b}=2{V}_{a}$, ${P}_{b}={P}_{a}$, ${T}_{b}=2{T}_{a}$
- c点:${V}_{C}={V}_{b}$, ${P}_{c}=\dfrac {1}{2}{P}_{a}$, ${T}_{c}={T}_{a}$
步骤 2:计算各过程中的功
- A→B等压过程:${A}_{1}=v{C}_{ma}({T}_{b}-{T}_{c})=\dfrac {7}{2}\times 8.31\times 48.8=1419.31J$
- B→C等容过程:${A}_{2}=0$
- C→A等温过程:${A}_{3}=vRTi\ln \dfrac {{v}_{1}}{{v}_{2}}=8.31\times 48.8\times (-0.693)=-281.0J$
步骤 3:计算整个循环过程的净功
- 整个循环过程的净功为:$A={A}_{1}+{A}_{2}+{A}_{3}=(405.2-281.0)J=124.2J$
步骤 4:计算循环效率
- 循环效率为:$\eta =\dfrac {A}{Q}=\dfrac {124.2}{1419.3}=8.75\%$