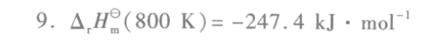题目
9.反应 _(2)(g)+dfrac (1)(2)(O)_(2)(g)=!=!= (H)_(2)O(1), 在298K时,反应热为 -285.84kJcdot (mol)^-1-|||-试计算反应在800K的热效应 (Delta )_(t)(H)_(m)^theta (800k) 已知:H2O(1)在373 K、p^θ时的蒸发热为-|||-.65kJcdot (mol)^-1;-|||-(({H)_(2))}^2((H)_(2))=29.07-0.84times (10)^-3T/K-|||-_(p,m)((O)_(2))=36.16+0.85times (10)^-3T/K-|||-_({P)_(1)m}((H)_(2)O,1)=75.26-|||-_(pm)((H)_(2)O,g)=30.0+10.71times (10)^-3T/K-|||-CB.m单位均为 cdot Kcdot (mol)^-1, 等式左边均除以该量纲。

题目解答
答案