题目
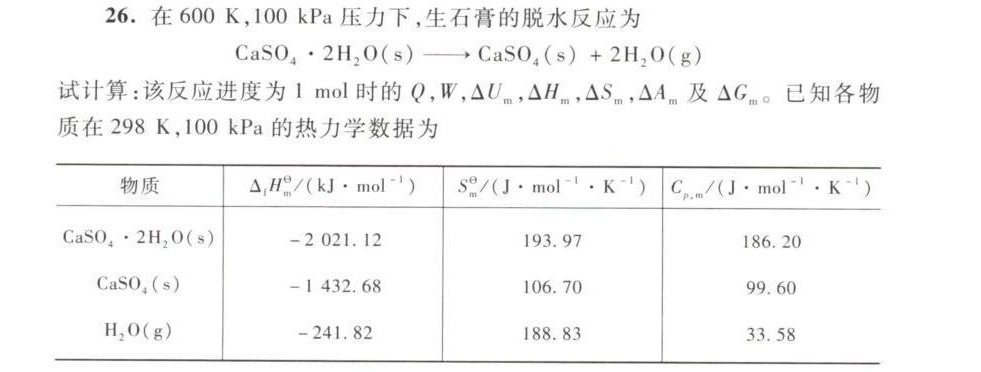
26.在600K,100 kPa压力下,生石膏的脱水反应为-|||-(O)_(4)cdot 2(H)_(2)O(s)arrow CaS(O)_(4)(s)+2(H)_(2)O(g)-|||-试计算:该反应进度为1mol时的Q,W, Delta (L)_(m), Delta (H)_(m) Delta (S)_(m) Delta (A)_(m) 及 △Gm。 已知各物-|||-质在298K,100 kPa的热力学数据为-|||-物质 (Delta )_(1)(H)_(m)^theta /(kJcdot mo(l)^-1) _(m)^theta /(1cdot mo(l)^-1cdot (K)^-1) C_(1·mol^(-1)·K^(-1))-|||-CaSO4·2H2O(s) -2021.12 193.97 186.20-|||-CaSO4(s) -1432.68 106.70 99.60-|||-H2O(g) -241.82 188.83 33.58
题目解答
答案
