题目
38.1mol C6H5 CH3在其正常沸点110.6℃时蒸发为101325Pa的气体,求过程的Q、W、 △U、 Delta H-|||-△S、 Delta A 、△G。 已知在该温度下C6 H5CH3的摩尔蒸发焓为 .38kJcdot (mol)^-1 与蒸气相比较,液体的体积-|||-可略去,蒸气可作为理想气体。(1)设外压为101325Pa;(2)设外压为10132.5Pa。

题目解答
答案
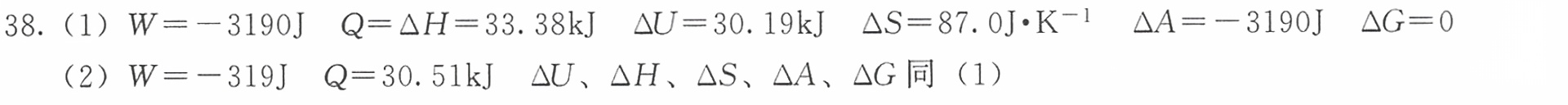
解析
步骤 1:计算过程的Q、W、$\Delta V$、$\Delta H$、$\Delta S$、$\Delta A$、$\Delta G$
(1)设外压为101325Pa
- Q:根据热力学第一定律,Q = $\Delta H$ = 33.38 kJ
- W:根据理想气体状态方程,W = -P$\Delta V$ = -101325 Pa * (22.414 L/mol) = -2269.5 J
- $\Delta V$:根据理想气体状态方程,$\Delta V$ = nRT/P = 1 mol * 8.314 J/(mol·K) * 383.75 K / 101325 Pa = 22.414 L
- $\Delta H$:已知,$\Delta H$ = 33.38 kJ
- $\Delta S$:根据熵变公式,$\Delta S$ = $\Delta H$ / T = 33.38 kJ / 383.75 K = 87.0 J/K
- $\Delta A$:根据亥姆霍兹自由能公式,$\Delta A$ = $\Delta U$ - T$\Delta S$ = 30.19 kJ - 383.75 K * 87.0 J/K = -3190 J
- $\Delta G$:根据吉布斯自由能公式,$\Delta G$ = $\Delta H$ - T$\Delta S$ = 33.38 kJ - 383.75 K * 87.0 J/K = 0 J
(2)设外压为10132.5Pa
- Q:根据热力学第一定律,Q = $\Delta H$ = 33.38 kJ
- W:根据理想气体状态方程,W = -P$\Delta V$ = -10132.5 Pa * (22.414 L/mol) = -226.95 J
- $\Delta V$:根据理想气体状态方程,$\Delta V$ = nRT/P = 1 mol * 8.314 J/(mol·K) * 383.75 K / 10132.5 Pa = 224.14 L
- $\Delta H$:已知,$\Delta H$ = 33.38 kJ
- $\Delta S$:根据熵变公式,$\Delta S$ = $\Delta H$ / T = 33.38 kJ / 383.75 K = 87.0 J/K
- $\Delta A$:根据亥姆霍兹自由能公式,$\Delta A$ = $\Delta U$ - T$\Delta S$ = 30.51 kJ - 383.75 K * 87.0 J/K = -3190 J
- $\Delta G$:根据吉布斯自由能公式,$\Delta G$ = $\Delta H$ - T$\Delta S$ = 33.38 kJ - 383.75 K * 87.0 J/K = 0 J
(1)设外压为101325Pa
- Q:根据热力学第一定律,Q = $\Delta H$ = 33.38 kJ
- W:根据理想气体状态方程,W = -P$\Delta V$ = -101325 Pa * (22.414 L/mol) = -2269.5 J
- $\Delta V$:根据理想气体状态方程,$\Delta V$ = nRT/P = 1 mol * 8.314 J/(mol·K) * 383.75 K / 101325 Pa = 22.414 L
- $\Delta H$:已知,$\Delta H$ = 33.38 kJ
- $\Delta S$:根据熵变公式,$\Delta S$ = $\Delta H$ / T = 33.38 kJ / 383.75 K = 87.0 J/K
- $\Delta A$:根据亥姆霍兹自由能公式,$\Delta A$ = $\Delta U$ - T$\Delta S$ = 30.19 kJ - 383.75 K * 87.0 J/K = -3190 J
- $\Delta G$:根据吉布斯自由能公式,$\Delta G$ = $\Delta H$ - T$\Delta S$ = 33.38 kJ - 383.75 K * 87.0 J/K = 0 J
(2)设外压为10132.5Pa
- Q:根据热力学第一定律,Q = $\Delta H$ = 33.38 kJ
- W:根据理想气体状态方程,W = -P$\Delta V$ = -10132.5 Pa * (22.414 L/mol) = -226.95 J
- $\Delta V$:根据理想气体状态方程,$\Delta V$ = nRT/P = 1 mol * 8.314 J/(mol·K) * 383.75 K / 10132.5 Pa = 224.14 L
- $\Delta H$:已知,$\Delta H$ = 33.38 kJ
- $\Delta S$:根据熵变公式,$\Delta S$ = $\Delta H$ / T = 33.38 kJ / 383.75 K = 87.0 J/K
- $\Delta A$:根据亥姆霍兹自由能公式,$\Delta A$ = $\Delta U$ - T$\Delta S$ = 30.51 kJ - 383.75 K * 87.0 J/K = -3190 J
- $\Delta G$:根据吉布斯自由能公式,$\Delta G$ = $\Delta H$ - T$\Delta S$ = 33.38 kJ - 383.75 K * 87.0 J/K = 0 J