题目
La2(C2O4)3的饱和溶液中,La2(C2 O4)3的浓度为 https:/img.zuoyebang.cc/zyb_cf986f45db9a4dcc0220775e72bd3fc8.jpg.1times (10)^-6molcdot (dm)^-3, 该化-|||-合物的溶度积常数为 () 。-|||-(A) https:/img.zuoyebang.cc/zyb_cf986f45db9a4dcc0220775e72bd3fc8.jpg.2times (10)^-12 (B) https:/img.zuoyebang.cc/zyb_cf986f45db9a4dcc0220775e72bd3fc8.jpg.6times (10)^-30 (C) https:/img.zuoyebang.cc/zyb_cf986f45db9a4dcc0220775e72bd3fc8.jpg.6times (10)^-34 (D) https:/img.zuoyebang.cc/zyb_cf986f45db9a4dcc0220775e72bd3fc8.jpg.7times (10)^-28

题目解答
答案
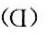
解析
步骤 1:确定溶度积常数的表达式
溶度积常数(Ksp)是描述难溶电解质在溶液中溶解度的常数。对于La2(C2O4)3,其溶度积常数表达式为:
\[ K_{sp} = [La^{3+}]^2[C_2O_4^{2-}]^3 \]
步骤 2:确定离子浓度
La2(C2O4)3在水中解离为La3+和C2O42-离子。根据化学式,每摩尔La2(C2O4)3解离产生2摩尔La3+和3摩尔C2O42-。因此,如果La2(C2O4)3的浓度为$1.1\times {10}^{-6}mol\cdot {dm}^{-3}$,则:
\[ [La^{3+}] = 2 \times 1.1\times {10}^{-6}mol\cdot {dm}^{-3} = 2.2\times {10}^{-6}mol\cdot {dm}^{-3} \]
\[ [C_2O_4^{2-}] = 3 \times 1.1\times {10}^{-6}mol\cdot {dm}^{-3} = 3.3\times {10}^{-6}mol\cdot {dm}^{-3} \]
步骤 3:计算溶度积常数
将离子浓度代入溶度积常数表达式中:
\[ K_{sp} = (2.2\times {10}^{-6})^2 \times (3.3\times {10}^{-6})^3 \]
\[ K_{sp} = 4.84\times {10}^{-12} \times 3.5937\times {10}^{-17} \]
\[ K_{sp} = 1.72\times {10}^{-28} \]
溶度积常数(Ksp)是描述难溶电解质在溶液中溶解度的常数。对于La2(C2O4)3,其溶度积常数表达式为:
\[ K_{sp} = [La^{3+}]^2[C_2O_4^{2-}]^3 \]
步骤 2:确定离子浓度
La2(C2O4)3在水中解离为La3+和C2O42-离子。根据化学式,每摩尔La2(C2O4)3解离产生2摩尔La3+和3摩尔C2O42-。因此,如果La2(C2O4)3的浓度为$1.1\times {10}^{-6}mol\cdot {dm}^{-3}$,则:
\[ [La^{3+}] = 2 \times 1.1\times {10}^{-6}mol\cdot {dm}^{-3} = 2.2\times {10}^{-6}mol\cdot {dm}^{-3} \]
\[ [C_2O_4^{2-}] = 3 \times 1.1\times {10}^{-6}mol\cdot {dm}^{-3} = 3.3\times {10}^{-6}mol\cdot {dm}^{-3} \]
步骤 3:计算溶度积常数
将离子浓度代入溶度积常数表达式中:
\[ K_{sp} = (2.2\times {10}^{-6})^2 \times (3.3\times {10}^{-6})^3 \]
\[ K_{sp} = 4.84\times {10}^{-12} \times 3.5937\times {10}^{-17} \]
\[ K_{sp} = 1.72\times {10}^{-28} \]