题目
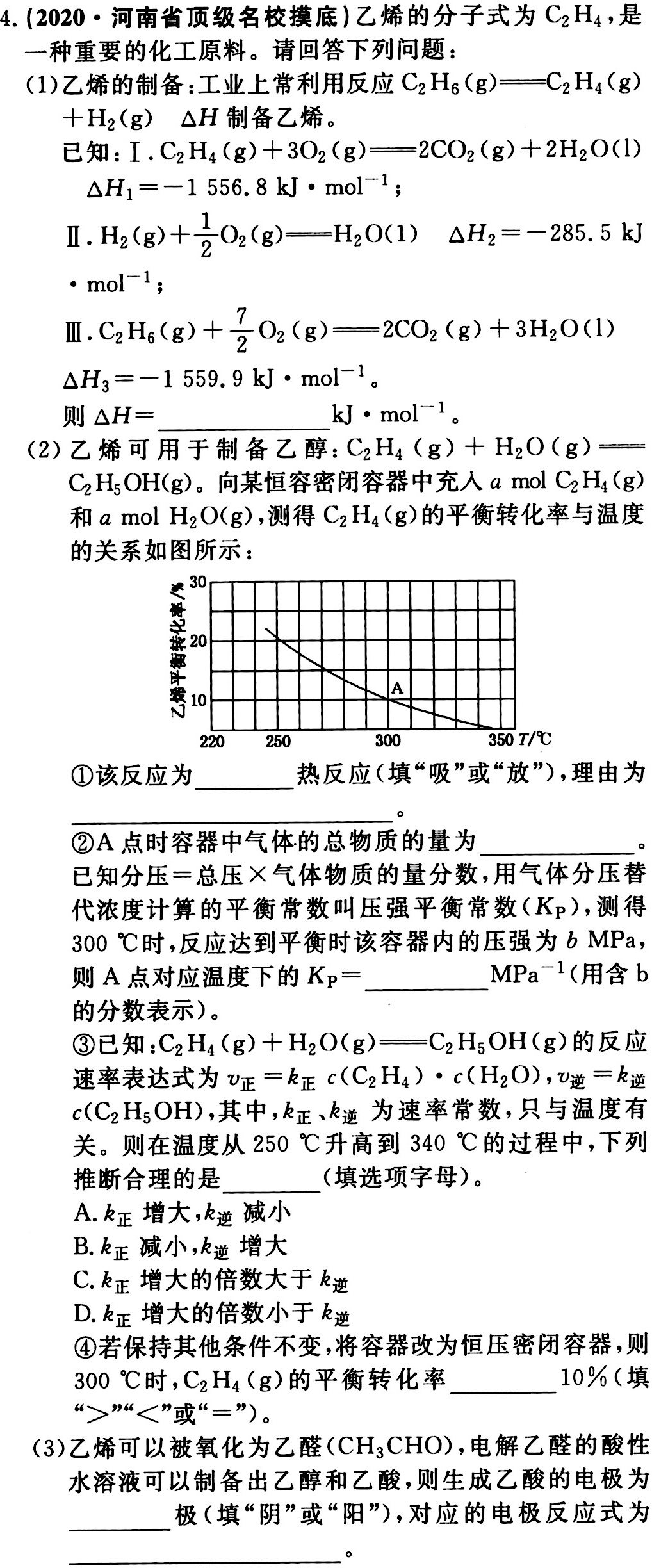
4.(2020·河南省顶级名校摸底)乙烯的分子式为C2H4,是-|||-一种重要的化工原料。请回答下列问题:-|||-(1)乙烯的制备:工业上常利用反应 _(2)(H)_(6)(g)=(C)_(2)(H)_(4)(g)-|||-+(H)_(2)(g) Delta H 制备乙烯。-|||-已知: .(C)_(2)(H)_(4)(g)+3(O)_(2)(g)=!=!= 2C(O)_(2)(g)+2(H)_(2)O(l)-|||-Delta (H)_(1)=-1556.8kJcdot (mol)^-1;-|||-Ⅱ. _(2)(g)+dfrac (1)(2)(O)_(2)(g)=!=!= (H)_(2)O(1) Delta (H)_(2)=-285.5kJ-|||-cdot (mol)^-1;-|||-Ⅲ. _(2)(H)_(6)(g)+dfrac (7)(2)(O)_(2)(g)=!=!= 2C(O)_(2)(g)+3(H)_(2)O(l)-|||-Delta (H)_(3)=-1559.9kJcdot (mol)^-1-|||-则 Delta H= __ cdot (mol)^-1-|||-__-|||-(2)乙烯可用于制备乙醇: _(2)(H)_(4)(g)+(H)_(2)O(g)=!=!= -|||-__-|||-C2H5OH(g)。向某恒容密闭容器中充入a mol C2H4(g)-|||-和amolH2O(g),测得C2H4(g)的平衡转化率与温度-|||-的关系如图所示:-|||-30-|||-20-|||-10 A-|||-220 250 300 350T/℃-|||-①该反应为 __ 热反应(填"吸"或"放"),理由为-|||-__-|||-②A点时容器中气体的总物质的量为 __-|||-已知分压=总压×气体物质的量分数,用气体分压替-|||-代浓度计算的平衡常数叫压强平衡常数(Kp),测得-|||-300℃时,反应达到平衡时该容器内的压强为bMPa,-|||-则A点对应温度下的 _(p)= __ (Pa)^-1 (用含b-|||-的分数表示)。-|||-③已知: _(2)(H)_(4)(g)+(H)_(2)O(g)=!=!= (C)_(2)(H)_(5)OH(g) 的反应-|||-速率表达式为 _(正)=(k)_(正)c((C)_(2)(H)_(4))cdot c((H)_(2)O), _(UND)=(k)_(UND)-|||-c(C2H5OH),其中,k正、k逆为速率常数,只与温度有-|||-关。则在温度从250℃升高到340℃的过程中,下列-|||-推断合理的是 __ (填选项字母)。-|||-A.k正增大,k逆减小-|||-B.k正减小,k逆增大-|||-C.k正增大的倍数大于k逆-|||-D.k正增大的倍数小于k逆-|||-④若保持其他条件不变,将容器改为恒压密闭容器,则-|||-300℃时,C2H4(g)的平衡转化率 __ 10%(填-|||-">""
题目解答
答案
