题目
15.已知反应:-|||-dfrac (1)(2)(H)_(2)(g)+dfrac (1)(2)(Cl)_(2)(g)leftharpoons HCl(g)-|||-在298.15K时的 _(1)^theta =4.9times (10)^16 _(1)(H)_(m)(298.15k)=-92.31kJcdot mo(l)^-1 求在500K时的 K2/2
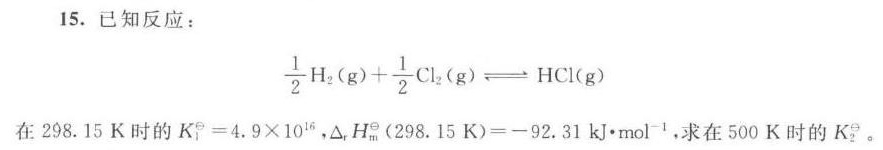
题目解答
答案
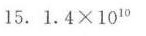
解析
步骤 1:确定反应的热力学数据
已知反应在298.15K时的平衡常数 ${K}_{1}^{\theta }=4.9\times {10}^{16}$ 和标准摩尔焓变 ${\Delta }_{t}{H}_{m}^{\theta }=-92.31kJ\cdot {mol}^{-1}$。
步骤 2:使用范特霍夫方程计算500K时的平衡常数
范特霍夫方程为:$\ln \left(\dfrac{{K}_{2}^{\theta }}{{K}_{1}^{\theta }}\right)=-\dfrac{{\Delta }_{t}{H}_{m}^{\theta }}{R}\left(\dfrac{1}{{T}_{2}}-\dfrac{1}{{T}_{1}}\right)$
其中,${T}_{1}=298.15K$,${T}_{2}=500K$,$R=8.314J\cdot {mol}^{-1}\cdot {K}^{-1}$。
步骤 3:代入数据计算
将已知数据代入范特霍夫方程,计算500K时的平衡常数 ${K}_{2}^{\theta }$。
$\ln \left(\dfrac{{K}_{2}^{\theta }}{4.9\times {10}^{16}}\right)=-\dfrac{-92.31\times {10}^{3}J\cdot {mol}^{-1}}{8.314J\cdot {mol}^{-1}\cdot {K}^{-1}}\left(\dfrac{1}{500K}-\dfrac{1}{298.15K}\right)$
$\ln \left(\dfrac{{K}_{2}^{\theta }}{4.9\times {10}^{16}}\right)=\dfrac{92.31\times {10}^{3}}{8.314}\left(\dfrac{1}{298.15}-\dfrac{1}{500}\right)$
$\ln \left(\dfrac{{K}_{2}^{\theta }}{4.9\times {10}^{16}}\right)=11.09$
$\dfrac{{K}_{2}^{\theta }}{4.9\times {10}^{16}}={e}^{11.09}$
${K}_{2}^{\theta }=4.9\times {10}^{16}\times {e}^{11.09}$
${K}_{2}^{\theta }=1.4\times {10}^{10}$
已知反应在298.15K时的平衡常数 ${K}_{1}^{\theta }=4.9\times {10}^{16}$ 和标准摩尔焓变 ${\Delta }_{t}{H}_{m}^{\theta }=-92.31kJ\cdot {mol}^{-1}$。
步骤 2:使用范特霍夫方程计算500K时的平衡常数
范特霍夫方程为:$\ln \left(\dfrac{{K}_{2}^{\theta }}{{K}_{1}^{\theta }}\right)=-\dfrac{{\Delta }_{t}{H}_{m}^{\theta }}{R}\left(\dfrac{1}{{T}_{2}}-\dfrac{1}{{T}_{1}}\right)$
其中,${T}_{1}=298.15K$,${T}_{2}=500K$,$R=8.314J\cdot {mol}^{-1}\cdot {K}^{-1}$。
步骤 3:代入数据计算
将已知数据代入范特霍夫方程,计算500K时的平衡常数 ${K}_{2}^{\theta }$。
$\ln \left(\dfrac{{K}_{2}^{\theta }}{4.9\times {10}^{16}}\right)=-\dfrac{-92.31\times {10}^{3}J\cdot {mol}^{-1}}{8.314J\cdot {mol}^{-1}\cdot {K}^{-1}}\left(\dfrac{1}{500K}-\dfrac{1}{298.15K}\right)$
$\ln \left(\dfrac{{K}_{2}^{\theta }}{4.9\times {10}^{16}}\right)=\dfrac{92.31\times {10}^{3}}{8.314}\left(\dfrac{1}{298.15}-\dfrac{1}{500}\right)$
$\ln \left(\dfrac{{K}_{2}^{\theta }}{4.9\times {10}^{16}}\right)=11.09$
$\dfrac{{K}_{2}^{\theta }}{4.9\times {10}^{16}}={e}^{11.09}$
${K}_{2}^{\theta }=4.9\times {10}^{16}\times {e}^{11.09}$
${K}_{2}^{\theta }=1.4\times {10}^{10}$