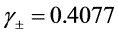题目
8电池(s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s),测得25℃时电动势(s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s)。已知:(s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s),(s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s)。(1)写出电池反应(得失电子数为2);(2)求上述反应的标准平衡常数(s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s);(3)求溶液(s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s)的平均离子活度因子(s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s)。For cell(s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s), at 25℃(s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s).Given(s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s),(s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s).(1)Write out cell reaction ((s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s))(2)Calculate (s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s)of the above cell reaction .(3)Calculate (s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s)of the (s)|Zn(Cl)_(2)(0.555molcdot (Kg)^-1)|AgCl(s)|Ag(s) solution.
8电池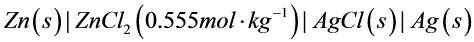 ,测得25℃时电动势
,测得25℃时电动势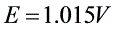 。已知:
。已知: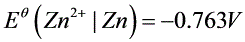 ,
,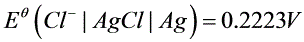 。
。
(1)写出电池反应(得失电子数为2);
(2)求上述反应的标准平衡常数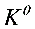 ;
;
(3)求溶液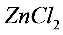 的平均离子活度因子
的平均离子活度因子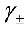 。
。
For cell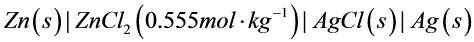 , at 25℃
, at 25℃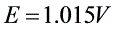 .Given
.Given
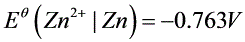 ,
,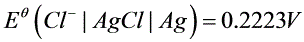 .
.
(1)Write out cell reaction (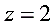 )
)
(2)Calculate 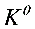 of the above cell reaction .
of the above cell reaction .
(3)Calculate 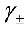 of the
of the 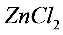 solution.
solution.
题目解答
答案
(1)Zn+2AgCl(s) === ZnCl2(b=0.555mol·kg1)+2Ag;(2) 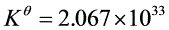
(3)