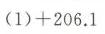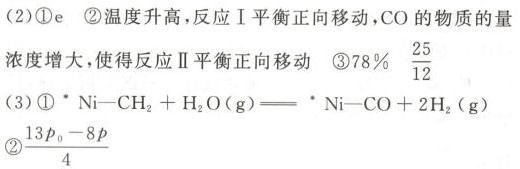题目
Delta (H)_(1)=akJcdot (mol)^-1-|||-反应Ⅱ: (g)+(H)_(2)O(g)leftharpoons C(O)_(2)(g)+(H)_(2)(g)-|||-Delta (H)_(2)=-41kJcdot (mol)^-1-|||-回答下列问题:-|||-(1)标准摩尔生成焓( Delta (H)^+(m)^6 )是指标准状态-|||-下,由稳定的单质生成1mol该物质的焓变。-|||-稳定单质的 (Delta )_(1)(H)_(m)^theta =0kJcdot (mol)^-1 。根据下表-|||-数据计算 a= __ o-|||-物质 CH4(g) CO(g) H2O(g)-|||-(Delta )_(1)(H)_(m)^theta /kJcdot (mol)^-1 -74.8 -110.5 -241.8-|||-(2)向恒容密闭容器中按 (C(H)_(4)):n((H)_(2)O)=-|||-1:3投料,初始总压强为p0,测得平衡时各组分-|||-的物质的量分数与温度的关系如图所示。-|||-乘0.6-|||-a-|||-0.5 N(600,0.5-|||-当0.4-|||-0.3 b-|||-0.2-|||-c-|||-0.1 d e-|||-M(600,0.04)-|||-0-|||-500 600 700 800-|||-温度/℃-|||-①图中表示CO的物质的量分数随温度变化-|||-的曲线是 __ 。-|||-②温度低于600℃时,d的物质的量分数随温-|||-度升高而增大,原因是 __Delta (H)_(1)=akJcdot (mol)^-1-|||-反应Ⅱ: (g)+(H)_(2)O(g)leftharpoons C(O)_(2)(g)+(H)_(2)(g)-|||-Delta (H)_(2)=-41kJcdot (mol)^-1-|||-回答下列问题:-|||-(1)标准摩尔生成焓( Delta (H)^+(m)^6 )是指标准状态-|||-下,由稳定的单质生成1mol该物质的焓变。-|||-稳定单质的 (Delta )_(1)(H)_(m)^theta =0kJcdot (mol)^-1 。根据下表-|||-数据计算 a= __ o-|||-物质 CH4(g) CO(g) H2O(g)-|||-(Delta )_(1)(H)_(m)^theta /kJcdot (mol)^-1 -74.8 -110.5 -241.8-|||-(2)向恒容密闭容器中按 (C(H)_(4)):n((H)_(2)O)=-|||-1:3投料,初始总压强为p0,测得平衡时各组分-|||-的物质的量分数与温度的关系如图所示。-|||-乘0.6-|||-a-|||-0.5 N(600,0.5-|||-当0.4-|||-0.3 b-|||-0.2-|||-c-|||-0.1 d e-|||-M(600,0.04)-|||-0-|||-500 600 700 800-|||-温度/℃-|||-①图中表示CO的物质的量分数随温度变化-|||-的曲线是 __ 。-|||-②温度低于600℃时,d的物质的量分数随温-|||-度升高而增大,原因是 __
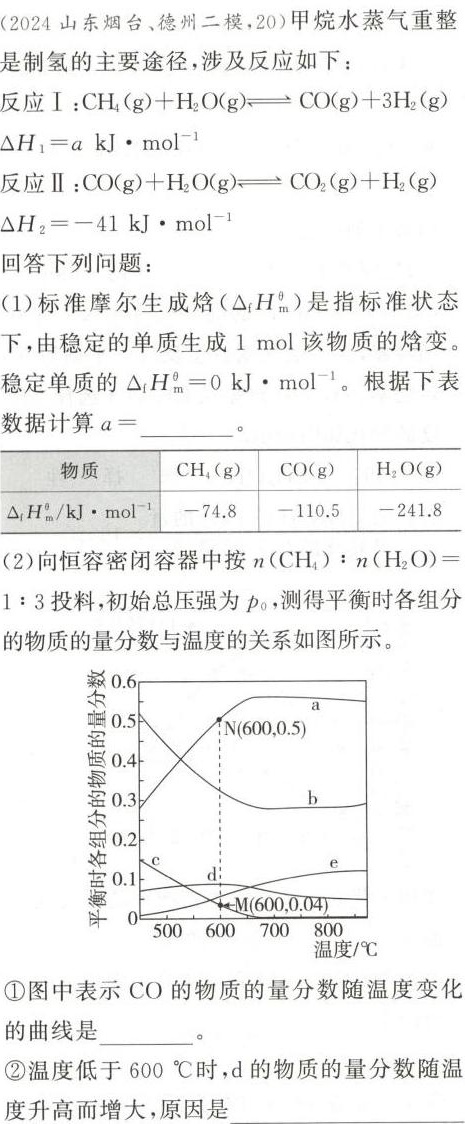

题目解答
答案