题目
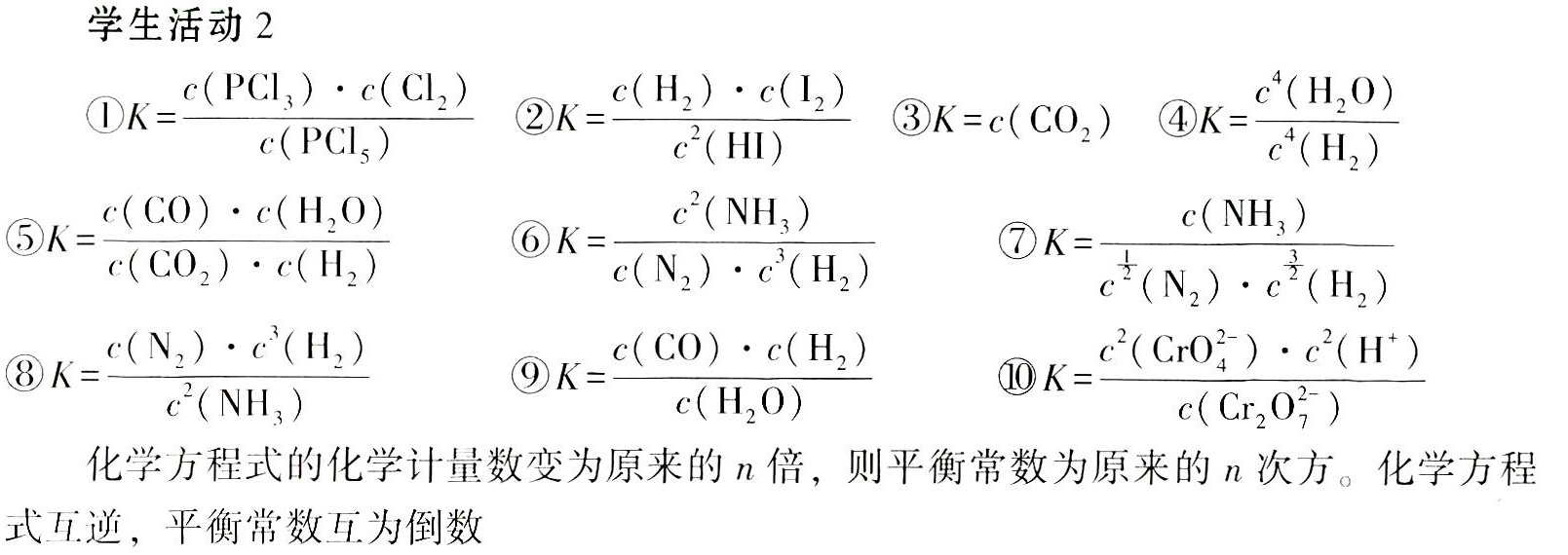
写出下列反应的平衡常数的表达式。-|||-① (Cl)_(5)(g)leftharpoons P(Cl)_(3)(g)+(Cl)_(2)(g)-|||-② (g)leftharpoons (H)_(2)(g)+(I)_(2)(g)-|||-③ (O)_(3)(s)leftharpoons CuO(s)+C(O)_(2)(g)-|||-④ _(3)(O)_(4)(s)+4(H)_(2)(g)leftharpoons 3Fe(s)+4(H)_(2)O(g)-|||-⑤ (O)_(2)(g)+(H)_(2)(g)=!=!= CO(g)+(H)_(2)O(g)-|||-⑥ _(2)(g)+3(H)_(2)(g)leftharpoons 2N(H)_(3)(g)-|||-⑦ dfrac (1)(2)(N)_(2)(g)+dfrac (3)(2)(H)_(2)(g)leftharpoons N(H)_(3)(g)-|||-⑧ (H)_(3)(g)leftharpoons (N)_(2)(g)+3(H)_(2)(g)-|||-⑨ (s)+(H)_(2)O(g)leftharpoons CO(g)+(H)_(2)(g)-|||-⑩ ({Cr)_(2)(O)_(7)}^2-+(H)_(2)Oleftharpoons 2(CrO)^2-+2(H)^+-|||-试从⑥⑦⑧分析平衡常数的表达式与化学方程式的关系:化学方程式的化学计-|||-量数变为原来的n倍,则平衡常数为原来的n次方。化学方程式互逆,平衡常数互为倒数

题目解答
答案
 X
X